INSTITUTIONAL BIOSAFETY COMMITTEE (IBC), UMS
Introduction and Background
Biosafety Act 2007 and Biosafety Regulation (Approval & Notification) 2010
The function and objectives of UMS IBC are:
-
To accord relevant exposure to GMO researchers in UMS on the importance of biosafety issues pertaining to the use of GMO/recombinant DNA directly relating to their research, health of their workers and the safety of the community.
-
To review and monitor the research activities of GMO carried out at the university to ensure compliance with the Biosafety Act.
-
To receive applications for any GMO research to be carried out at the university.
-
To review and decide on all applications for GMO research and to advise the researchers accordingly on their application with regards to compliances to stated guidelines.
-
To monitor the physical infrastructure, working procedures, training and expertise of staff involved in the approved GMO research.
-
To report to the Vice Chancellor on any significant issues on GMO use in UMS or on non-complying researchers on the set guidelines.
-
To become the contact point between the National Biosafety Board Malaysia (NBB) and UMS.
-
To report on all GMO research applications received from UMS researchers to the NBB.
The members are UMS researchers who have relevant expertise in the field of recombinant DNA and/or biosafety. Members are appointed by UMS based on recommendation by the Deputy Vice Chancellor (Research & Innovation), UMS.
IBC members are not permanent positions and opportunity is given to UMS researches to contribute. The IBC Chair notifies NBB of changes in IBC membership as and when they occur. Such notice should include a revised list of members, contact details and background information on each new member.
Current UMS IBC members:
|
No. |
List of members |
Designation in IBC, UMS |
Department / Agency |
|
1 |
Prof. Dr. Vijay Kumar, FASc. |
Chairman |
Professor of Molecular Genetics Biotechnology Research Institute (BRI) |
|
2 |
Assoc. Prof. Dr. Kenneth F. Rodrigues |
Biosafety Officer |
Deputy Director (R&I), Biotechnology Research Institute |
|
3 |
Assoc. Prof. Dr. Cahyo Budiman |
Member |
Biotechnology Research Institute |
|
4 |
Assoc. Prof. Dr. Teoh Peik Lin |
Member |
Biotechnology Research Institute |
|
5 |
Assoc. Prof. Dr. Azwan Awang |
Member |
Dean, Faculty of Sustainable Agriculture |
|
6 |
Dr. Mohammad Tamrin bin Mohammad Lal |
Member |
Deputy Director (R&I), Borneo Marine Research Institute |
|
7 |
Dr. Fong Siat Yee@Alison |
Member |
Faculty of Medicine and Health Sciences |
|
8 |
Dr. Mohd. Khalizan bin Sabullah |
Member |
Faculty of Science and Natural Resources |
|
9 |
Mdm. Salmi Afidah Shabuddin (Ts.) |
Secretariat |
Biotechnology Research Institute |
Application to Conduct Recombinant DNA / Modern Biotechnology Research at UMS
Register Your Activities
Research activities that involved in the use and research of GMO research/recombinant DNA specifically involving modern biotechnology are required to registered via google form develop by IBC, UMS as follow:
IBC, UMS will advice and guide researchers accordingly with regards to compliances to Biosafety Act 2007 guidelines.
Notification For Contained Use
Definition of Contained Use
Any operation including R&D, production or manufacturing operation involving LMOs, or storage of LMOs, undertaken within a facility, installation or other physical structure such as it prevents contact and impact of the LMOs on the external environment.
Application for contained use may involve the following:
- Application to export of living modified organisms
- Contained use involving living modified organisms
- Importation of living modified organisms for purposes of undertaking a contained use activity
Submission of notification
The notification shall be submitted to the Director General through the Institutional Biosafety Committee in the prescribed form and accompanied by the following documents:
- An emergency response plan
- Specific measures for the contained use activity (regulation + IBC)
- Such other information as may be specified by the Board
- Compliant with requirements of importing country (export)
Notification Process
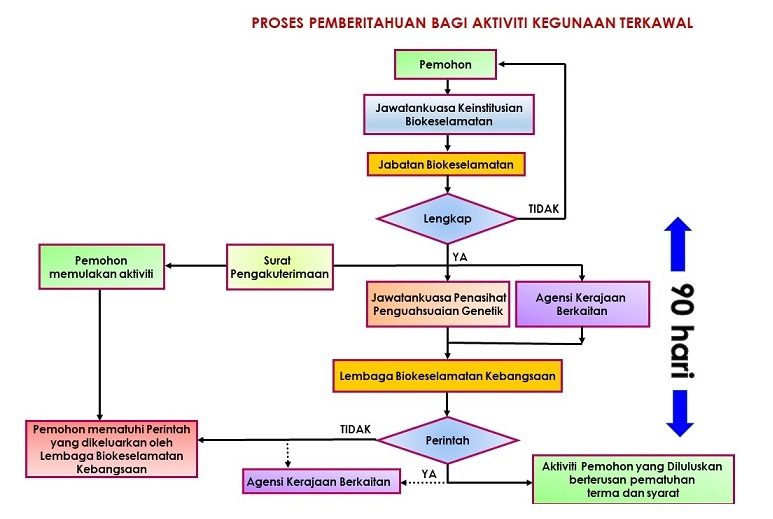
Application to Conduct Recombinant DNA / Modern Biotechnology Research at UMS
Approval
All release and import of LMOs and products of such is prohibited unless approved by the National Biosafety Board. The release activities as spelt out in the 3rd schedule in the Act are:
- Research and development purposes in all field experiments
- Supply or offer to supply for sale or placing on the market
- Offer as gift, prize or free item
- Disposal
- Remediation purposes
- Any other activity which does not amount to contained use
Approval Process
An application for approval must be completed and submitted to the Director General (DG), Biosafety Department, Ministry of Environment & Water in the prescribed manner, together with the prescribed fees, and be accompanied with:
- Risk assessment and a risk management report emergency
- Responses plan
- Other information as may be specified by the National Biosafety Board (NBB)
Upon receiving the application, the DG shall
- Refer it to Genetic Modification Advisory Committee (GMAC) for its recommendations
- Refer it to relevant government agencies for specific matters
- Invite public participation for purpose of public disclosure
GMAC shall forward its recommendation whether the application should be approved and the terms and conditions to be imposed by the NBB, if any, after the assessment.
After having considered the recommendations of the GMAC, the comments of the relevant department or agency, the views of members of the public, if any, and any additional information, the NBB may grant the application by issuing a certificate of approval or refuse the application.
If you have any questions, please contact:
Assoc. Prof. Dr. Kenneth F. Rodrigues (e-mail: kennethr@ums.edu.my) or
Mdm. Salmi Afidah Shabuddin (e-mail: salmi@ums.edu.my)
UMS Institutional Biosafety Committee,
Biotechnology Research Institute,
Universiti Malaysia Sabah
